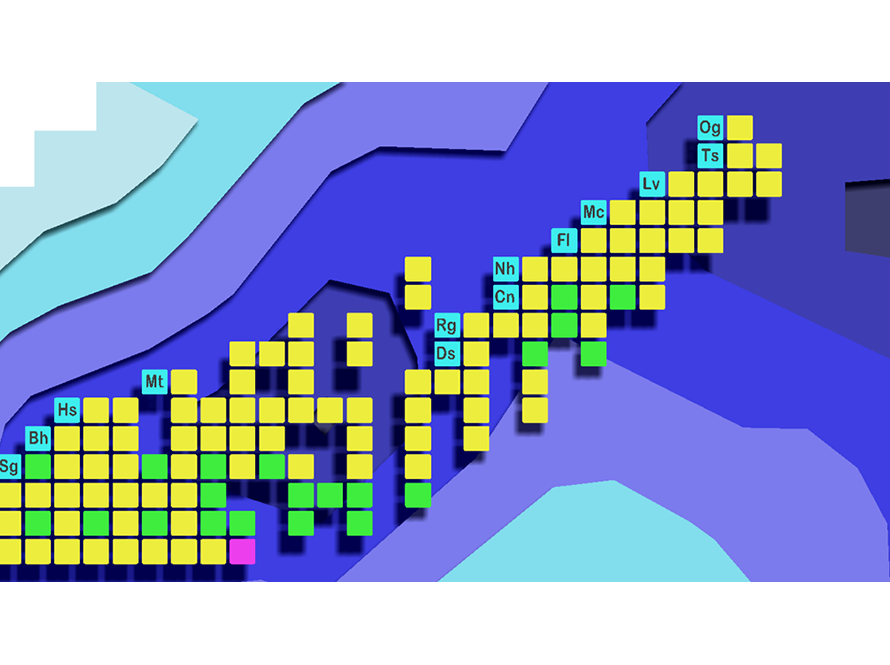
The main focus of our group is to study the heaviest elements known to man. These so-called Superheavy Elements do not exist naturally here on earth and are produced one atom at a time in nuclear reactions at accelerator facilities like the 88-Inch Cyclotron. Currently, we are investigating the best way to produce new elements, their decay properties, the number of protons and neutrons contained within their nuclei, how those protons and neutrons are arranged, how long superheavy elements live before decaying, and what chemical properties these superheavy elements have.
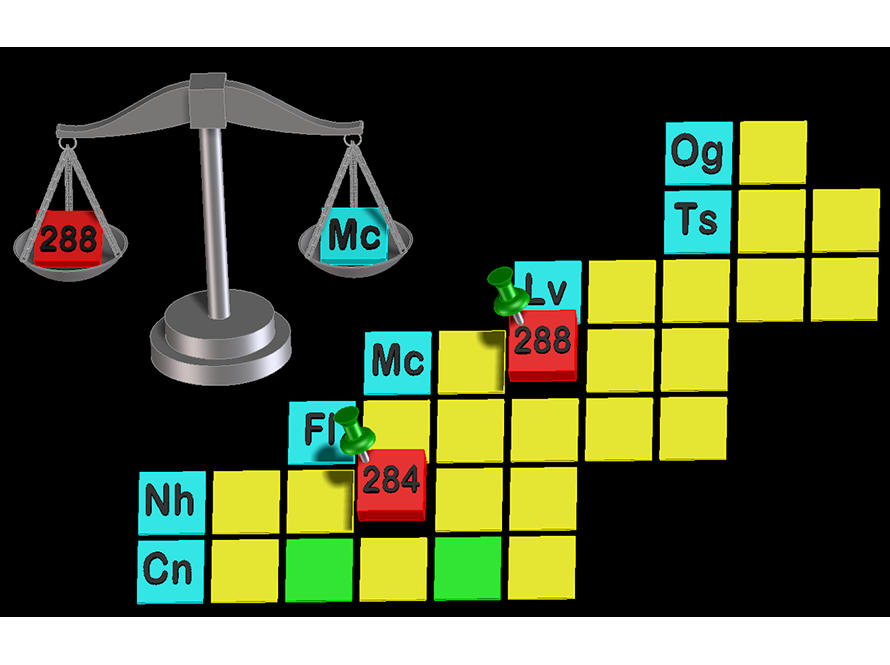
While scientists have added six new elements to the periodic table since 2010, we still know very little about these new elements. Even the number of protons and neutrons in their nuclei has largely been uninvestigated. Using our mass analyzer, FIONA, we successfully measured the mass of the first of these elements: Moscovium and Nihonium. Further experiments will work towards measuring the masses of Flerovium and Livermorium and discovering new isotopes.
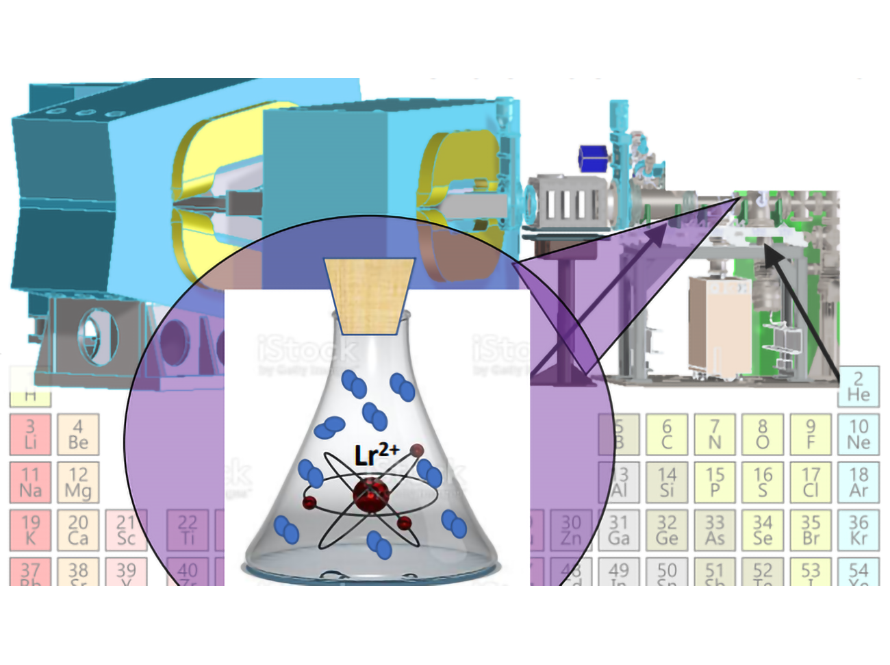
Very little is known about the chemical properties of the heaviest element as they are only produced one atom at a time and only live for seconds or even milliseconds before they decay into another element. With the introduction of the FIONA mass analyzer and its gas catcher, we are now able to study chemical reactions of superheavy elements in the gas phase. This allows us to determine the ionization potentials of superheavy elements, what compounds they make, and how fast they react chemically.
Neutron-deficient actinide elements have a unique trick: they can decay via electron-capture to high-lying states in the daughter nucleus that quickly fission, a process known as electron-capture-delayed fission, or ECDF. While we know this process exists, very little is known about the nuclei that undergo ECDF. We are working to understand this process in more detail and to discover new ECDF nuclides using our unique experimental facilities.
Our group has two spectroscopy stations that can be used to study the nuclear structure of heavy and superheavy elements. Doing this allows us to understand how the protons and neutrons in the nucleus are arranged, what combinations of protons and neutrons are especially stable (or unstable), and what different types of decay modes a superheavy element could undergo.
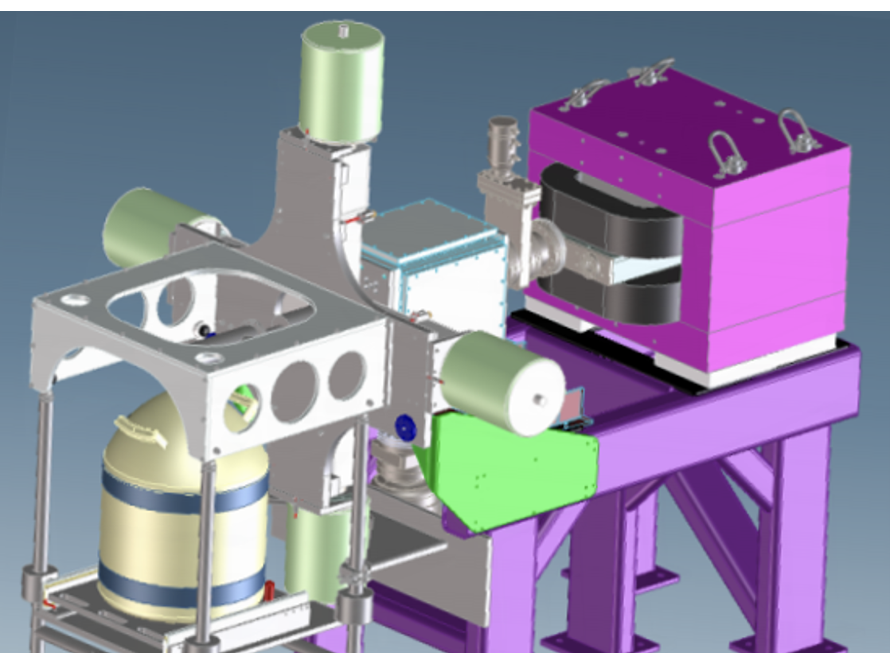
When nuclei undergo alpha decay, they often decay to excited states in the daughter nuclei. Those excited states can then deexcite via the emission of photons and electrons. We investigate these decays to learn more about the nuclear structure around the heaviest elements.
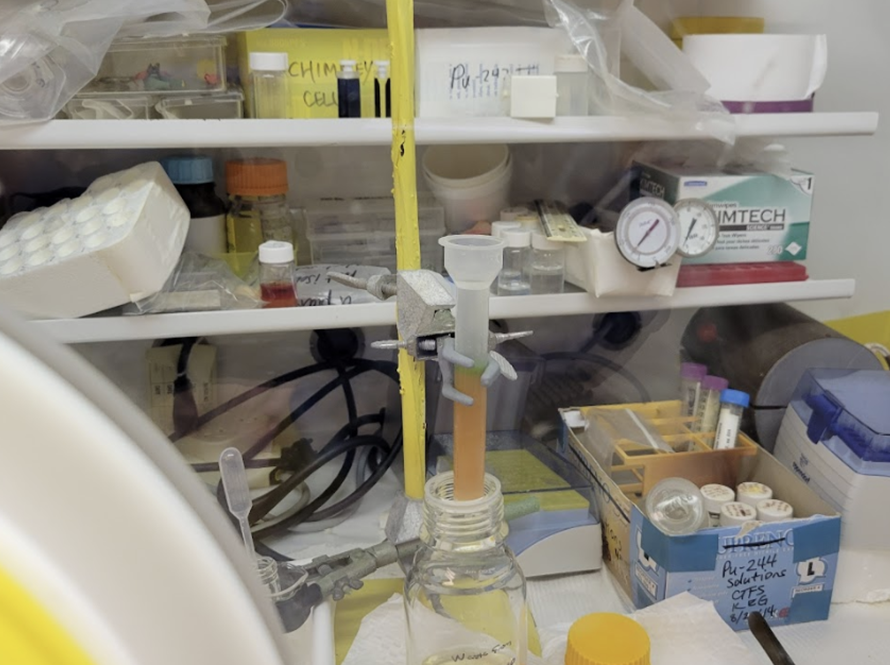
To make the heaviest known elements, we bombard actinide targets with 48Ca or 50Ti beams. As actinide elements are radioactive, producing these targets can only be done in select laboratories around the world. We have an in-house program to make actinide targets for superheavy element production using LBNL’s Heavy Element Radiation Laboratory.